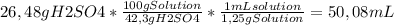Chemistry, 09.09.2019 19:10, runaway173
50 ml of a mixture consisting of 0.019 m cerium (iv) and 2.7 m h2so4, or sulfuric acid. begin by preparing 100 ml of 2.7 m h2so4 using the available solution of 42.3% w/w h2so4. this concentration unit, which may be less familiar to you, is a weight-to-weight percent (100.0 g of the solution contains 42.3 g of h2so4). the density of 42.3% w/w h2so4 is 1.25 g solution/ml solution. using a graduated cylinder, measure out the correct volume of 42.3% w/w h2so4 and slowly add it to a 100-ml volumetric flask that already contains approximately 25 ml of deionized water.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 17:30, cynthiagutierrez65
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1


Chemistry, 21.06.2019 23:50, ShlomoShekelstein
Why do scientists look for patterns in the world? a. patterns can explain observations. b. patterns never change, no matter what. c. patterns are easy for scientists to detect. d. patterns are all the same, through all time.
Answers: 1
Do you know the correct answer?
50 ml of a mixture consisting of 0.019 m cerium (iv) and 2.7 m h2so4, or sulfuric acid. begin by pre...
Questions in other subjects: