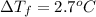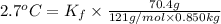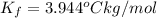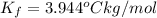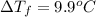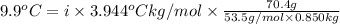Chemistry, 09.09.2019 18:30, chloejaylevesque
When 70.4 g of benzamide (c, h,no) are dissolved in 850. g of a certain mystery liquid x, the freezing point of the solution is 2.7 c lower than the freezing point of pure x. on the other hand, when 70.4 g of ammonium chloride (nh ci) are dissolved in the same mass of x, the freezing point of the solution is 9.9 °c lower than the freezing point of pure x. calculate the van't hoff factor for ammonium chloride in x. be sure your answer has a unit symbol, if necessary, and round your answer to 2 significant digits.

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 03:00, sharondacarruth1656
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1
Do you know the correct answer?
When 70.4 g of benzamide (c, h,no) are dissolved in 850. g of a certain mystery liquid x, the freezi...
Questions in other subjects:


English, 06.05.2020 09:02


Mathematics, 06.05.2020 09:02

Mathematics, 06.05.2020 09:02






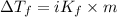
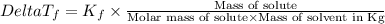 ...(1)
...(1)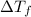 =Elevation in boiling point =
=Elevation in boiling point = 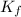 = Freezing point constant
= Freezing point constant