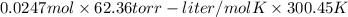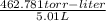Chemistry, 07.09.2019 05:30, dogsarecute278
The vapor pressure of a volatile liquid can be determined by slowly bubbling a known volume of gas through it at a known temperature and pressure. in an experiment, 5.01 l of n2 gas is passed through 7.9286 g of liquid benzene, c6h6, at 27.3 ∘c and atmospheric pressure. the liquid remaining after the experiment weighs 5.9987 g . assuming that the gas becomes saturated with benzene vapor and that the total gas volume and temperature remain constant, what is the vapor pressure of the benzene in torr?

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 22:00, allieeastridge
For a family dinner, jose’s mom baked a loaf of bread. during the meal, his grandmother commented, “this bread is so dense! ” what do you think his grandmother meant by the word dense? you have some ideas regarding density already, but this experiment will enrich your understanding of the relationship between mass, volume and the density of objects. when making measurements in this experiment we will be using si units, the international system of units. the purpose of using si units is to have the ability to communicate and compare data results with other experiments without having to make conversions. measurement symbol si unit length m meter mass kg kilogram volume l liters density kg/l kilograms per liter any calculations that are performed during this experiment will ultimately be reported in scientific notation. recall that scientific notation is extremely important when we have data that is extremely small or large. scientists rely on a standard form of communication to quickly make hypotheses and judgements based on data. what is the density of block a? a0kg/l what is the density of block b? a1kg/l what is the density of block c? a2kg/l what is the density of block d? a3kg/l what is the density of block e? a4kg/l which block is the densest? a5
Answers: 1

Chemistry, 23.06.2019 08:00, yddlex
Suppose a pair of chemical compounds a and b can react in two different ways: a + b -> c reaction 1 gives product c. a + b -> d reaction 2 gives product d. the following facts are known about the two reactions: . reaction 1 is endothermic and reaction 2 is exothermic. if a reaction vessel is charged (filled) with a and b , then at first d is produced faster than c. use these facts to sketch a qualitative reaction energy diagram for both reactions. note: because these sketches are only qualitative, the energies don? t have to be exact. they only have to have the right relationship to each other. for example, if one energy is less than another, that fact should be clear in your sketch.
Answers: 3
Do you know the correct answer?
The vapor pressure of a volatile liquid can be determined by slowly bubbling a known volume of gas t...
Questions in other subjects:




English, 11.11.2020 16:40



Physics, 11.11.2020 16:40


English, 11.11.2020 16:40


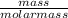
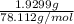
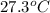 = 27.3 + 273.15 = 300.45 K
= 27.3 + 273.15 = 300.45 K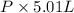 =
=