
Chemistry, 07.09.2019 02:30, sofiisabella10
For a particular reaction, δh∘=67.7 kj/molδh∘=67.7 kj/mol and δ∘=126.9 j/(mol⋅k).δs∘=126.9 j/(mol⋅k). assuming these values change very little with temperature, at what temperature does the reaction change from nonspontaneous to spontaneous in the forward direction?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, ashleyjaslin
Calculate the expected ph values of the buffer systems from the experiments (a, b,c, d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2


Chemistry, 23.06.2019 00:30, rose888829
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2
Do you know the correct answer?
For a particular reaction, δh∘=67.7 kj/molδh∘=67.7 kj/mol and δ∘=126.9 j/(mol⋅k).δs∘=126.9 j/(mol⋅k)...
Questions in other subjects:

Mathematics, 23.09.2020 18:01

Spanish, 23.09.2020 18:01

Mathematics, 23.09.2020 18:01

Mathematics, 23.09.2020 18:01


History, 23.09.2020 18:01





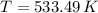
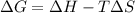
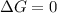 . If we insert that into our equation we get:
. If we insert that into our equation we get: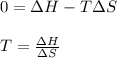
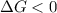 , thus the reaction will be spontaneous.
, thus the reaction will be spontaneous. 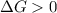 . That is, the reaction will not be spontaneous. Therefore for temperatures higher than 533.49 K we will see a spontaneous reaction, and for temperatures lower than that the reaction will not be spontaneous.
. That is, the reaction will not be spontaneous. Therefore for temperatures higher than 533.49 K we will see a spontaneous reaction, and for temperatures lower than that the reaction will not be spontaneous.



