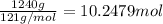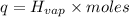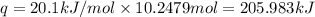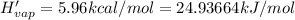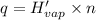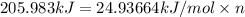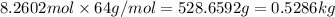Chemistry, 07.09.2019 01:30, krishimotam
Before the introduction of chlorofluorocarbons, sulfur dioxide (enthalpy of vaporization, 5.96 kcal/mol) was used in household refrigerators. what mass (in kg) of so2 must be evaporated to remove as much heat as evaporation of 1.24 kg of ccl2f2 (enthalpy of vaporization is 20.1 kj/mol)?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 02:00, Robloxdemonduckyt
As light moves from one material into the next, which of the following affects how much the light waves will refract, or bend? angle at which the ray strikes the medium color of the material density of the material temperature of the light wave
Answers: 2


Chemistry, 23.06.2019 07:30, 22emilyl530
Chris is about to do an experiment to measure the density of water at several temperatures. his teacher has him prepare and sign a safety contract before beginning the experiment. which term is mostlikely part of the safety contract
Answers: 3
Do you know the correct answer?
Before the introduction of chlorofluorocarbons, sulfur dioxide (enthalpy of vaporization, 5.96 kcal/...
Questions in other subjects:


Computers and Technology, 19.07.2019 23:20



Computers and Technology, 19.07.2019 23:20

Computers and Technology, 19.07.2019 23:20





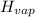 = 20.1 kJ/mol
= 20.1 kJ/mol