
(b) the conductivity of a 0.01 mol dm–3 solution of a monobasic organic acid in water is 5.07 × 10–2 s m–1. if the molar conductance at infinite dilution (λ°) of aqueous sodium chloride, sodium formate and hydrochloric acid are 1.264 × 10–2, 1.046 × 10–2 and 4.261 × 10–2 respectively at 25°c determine the acid dissociation constant and the pka for the acid.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 20:00, angeljohnson2081
Which object forms when a supergiant runs out of fuel? a red giant a black hole a white dwarf a neutron star
Answers: 1

Chemistry, 22.06.2019 01:00, bettybales1986
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Chemistry, 22.06.2019 06:30, yolo123321
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2
Do you know the correct answer?
(b) the conductivity of a 0.01 mol dm–3 solution of a monobasic organic acid in water is 5.07 × 10–2...
Questions in other subjects:







Mathematics, 26.10.2020 17:40



Mathematics, 26.10.2020 17:40


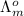 (NaCl) =
(NaCl) = 




 ) of monobasic acid is calculated as follows.
) of monobasic acid is calculated as follows.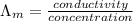




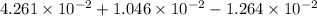
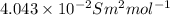
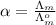
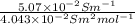

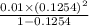

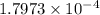
 and
and  is as follows.
is as follows.





