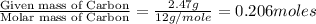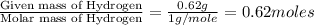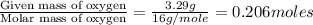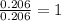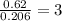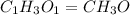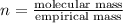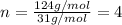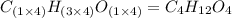Chemistry, 06.09.2019 23:20, zoewilliamss26
Ethylene glycol used in automobile antifreeze and in the production of polyester. the name glycol stems from the sweet taste of this poisonous compound. combustion of 6.38 g of this compound gives 9.06 g of co2 and 5.58 g of h2o. the compound only contains c, h, and o. if the molecular mass of this compound is 124 amu, what is the empirical and molecular formula?

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 19:00, georgesarkes12
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

Chemistry, 22.06.2019 21:30, Turtlelover05
How can the periodic table be used to predict the behavior of elements?
Answers: 1
Do you know the correct answer?
Ethylene glycol used in automobile antifreeze and in the production of polyester. the name glycol st...
Questions in other subjects:


Mathematics, 09.03.2021 19:00

Mathematics, 09.03.2021 19:00


Mathematics, 09.03.2021 19:00


English, 09.03.2021 19:00

Mathematics, 09.03.2021 19:00

Mathematics, 09.03.2021 19:00

Mathematics, 09.03.2021 19:00


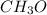 and
and 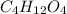
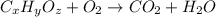
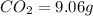
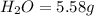
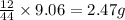 of carbon will be contained.
of carbon will be contained.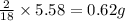 of hydrogen will be contained.
of hydrogen will be contained.