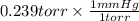Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 15:30, ricardotavarez6
How does a large body of water, such as the ocean, influence climate?
Answers: 1
Do you know the correct answer?
The partial pressure of carbon dioxide in the atmosphere is 0.239 torr. calculate the partial pressu...
Questions in other subjects:







English, 25.10.2021 16:10

History, 25.10.2021 16:10


Mathematics, 25.10.2021 16:10