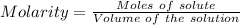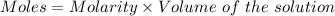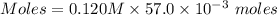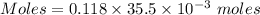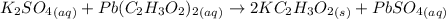Chemistry, 06.09.2019 22:10, loanyst99111
A57.0 ml sample of a 0.120 m potassium sulfate solution is mixed with 35.5 ml of a 0.118 m lead(ii) acetate solution and the following precipitation reaction occurs: k2so4(aq) pb(c2h3o2)2(aq)→2kc2h3o2(aq) pbso4(s) the solid pbso4 is collected, dried, and found to have a mass of 0.992 g . determine the limiting reactant, the theoretical yield, and the percent yield.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:40, draveon353
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 14:00, emilyproce
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 19:50, jakaylathomas11
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 23.06.2019 08:00, hardwick744
How does the digestive system interact with the circulatory system? a. messages sent as electrical impulses from the digestive system are transported throughout the body by the circulatory system. b. nutrients taken in and broken down by the digestive system are carried to various parts of the body by the circulatory system. c. nutrients and gases are absorbed by organs in the circulatory system. then, they are transported to all parts of the body by organs in the digestive system. d. oxygen and carbon dioxide are exchanged by organs in the digestive system, and the gases are carried to the rest of the body by the circulatory system.
Answers: 2
Do you know the correct answer?
A57.0 ml sample of a 0.120 m potassium sulfate solution is mixed with 35.5 ml of a 0.118 m lead(ii)...
Questions in other subjects:


Mathematics, 31.05.2021 22:40







Mathematics, 31.05.2021 22:40