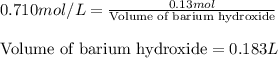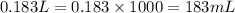Chemistry, 06.09.2019 22:10, zmoore8015
Calculate the number of milliliters of 0.710 m ba(oh)2 required to precipitate all of the mn2+ ions in 161 ml of 0.796 m kmno4 solution as mn(oh)2. the equation for the reaction is: mnso4(aq) + ba(oh)2(aq) mn(oh)2(s) + baso4(aq)

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, shifaxoxoxo
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 22.06.2019 12:00, Alexislol7908
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1
Do you know the correct answer?
Calculate the number of milliliters of 0.710 m ba(oh)2 required to precipitate all of the mn2+ ions...
Questions in other subjects:



Mathematics, 05.01.2020 04:31

Mathematics, 05.01.2020 04:31







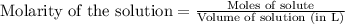 .....(1)
.....(1)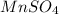 = 0.796 M
= 0.796 M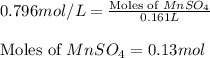
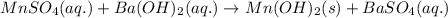
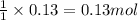 of barium hydroxide
of barium hydroxide