
Chemistry, 06.09.2019 21:20, villafana36
The complete oxidation of one mole of a sugar produces carbon dioxide and water. 2000 kj of heat is transferred from the system to the surroundings. the rearrangement of bonds as 0.5 moles of the sugar are oxidized generates heat in an open test tube (101 j•l–1 pressure and 300 k temperature). what is the change in internal energy of the system (δu)? what is the change in enthalpy of the system(δh)?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, 21brooklynmartin
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2

Chemistry, 22.06.2019 03:50, daniel9299
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 06:30, cadenhuggins2
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1
Do you know the correct answer?
The complete oxidation of one mole of a sugar produces carbon dioxide and water. 2000 kj of heat is...
Questions in other subjects:

History, 21.08.2019 10:20


Mathematics, 21.08.2019 10:20

Mathematics, 21.08.2019 10:20







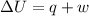
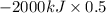
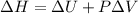
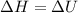
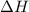 ) is -1000 kJ.
) is -1000 kJ.




