

Answers: 2
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 16:00, annsmith66
What statement goes against the kinetic theory of gases
Answers: 1
Do you know the correct answer?
Consider the reaction of cacn2 and water to produce caco3 and nh3: cacn2 + 3 h2o → caco3 + 2 nh3 ....
Questions in other subjects:



Mathematics, 01.07.2019 12:30



Mathematics, 01.07.2019 12:30

History, 01.07.2019 12:30

English, 01.07.2019 12:30

Mathematics, 01.07.2019 12:30

Mathematics, 01.07.2019 12:30


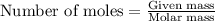
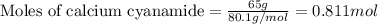
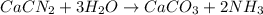
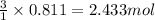 of water.
of water.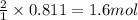 of ammonia.
of ammonia.



