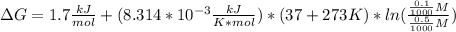Chemistry, 06.09.2019 20:30, angellynn581
2-phosphoglycerate(2pg) is converted to phosphoenolpyruvate (pep) by the enzyme enolase. the standard free energy change(deltago’) for this reaction is +1.7 kj/mol. if the cellular concentrations are 2pg = 0.5 mm and pep = 0.1 mm, what is the free energy change at 37 oc for the reaction 2pg ↔ pep
5.8 kj/mol
-5.8 kj/mol
+2.4 kj/mol
-2.4 kj/mol
-4146.4 kj/mol
+4146.4 kj/mol

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, mimibear2932
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 14:00, IdkHowToDoMath
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 14:30, amylumey2005
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2
Do you know the correct answer?
2-phosphoglycerate(2pg) is converted to phosphoenolpyruvate (pep) by the enzyme enolase. the standar...
Questions in other subjects:


Mathematics, 25.03.2020 22:38


Mathematics, 25.03.2020 22:38

Mathematics, 25.03.2020 22:38





Health, 25.03.2020 22:39


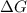 to
to 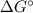 according to:
according to:![\Delta G=\Delta G\°+RTln(\frac{[products]^n}{[reagents]^m})](/tpl/images/0224/6819/0c5cf.png)