
Chemistry, 06.09.2019 18:30, nisazaheer
The common ion effect for ionic solids (salts) is to decrease the solubility of the ionic compound in water significantly. explain the common ion effect in detail.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:40, babygirlqueen5588
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3

Chemistry, 22.06.2019 10:00, sdlesley66
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 22.06.2019 14:50, rebeccamckellpidge
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 15:20, shanyeah
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1
Do you know the correct answer?
The common ion effect for ionic solids (salts) is to decrease the solubility of the ionic compound i...
Questions in other subjects:








Geography, 12.10.2020 17:01



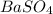
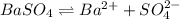
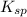 ) which is expressed as-
) which is expressed as-![K_{sp}=[Ba^{2+}][SO_{4}^{2-}]](/tpl/images/0224/5549/cd968.png)
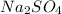 with a common ion
with a common ion 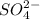 then concentration of
then concentration of 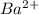 ion in solution and produce
ion in solution and produce 



