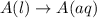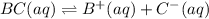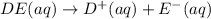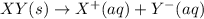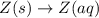Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:30, ayoismeisjjjjuan
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 19:00, elizabethajih99
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1
Do you know the correct answer?
Each of the following reactions shows a solute dissolved in water. classify each solute as a strong...
Questions in other subjects:

Mathematics, 07.07.2020 17:01

Physics, 07.07.2020 17:01


Mathematics, 07.07.2020 17:01

Mathematics, 07.07.2020 17:01

Mathematics, 07.07.2020 17:01


Biology, 07.07.2020 17:01