
Chemistry, 06.09.2019 17:30, wannamakerdaandre
Consider the general form of a reversible reaction to be: where the double arrow means the reaction can proceed in both the forward and reverse direction. if the equilibrium constant for the forward reaction is defined as: -, what is the equilibrium constant for the reverse reaction? a) it is exactly the same. namely b) it is just the products of the reactants. namely, c) it is equal to the reciprocal of the forward equilibrium constant. that is d) the reverse equilibrium constant cannot be determined. a) bi od) cl

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, Kaylinne1181
This chart represents the melting point of several substance. what besy explains the high melting point of the salt?
Answers: 2

Chemistry, 22.06.2019 01:00, chrisxxxrv24
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 15:30, 20cschultz
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 16:50, brandiwingard
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2
Do you know the correct answer?
Consider the general form of a reversible reaction to be: where the double arrow means the reaction...
Questions in other subjects:



Mathematics, 18.03.2020 21:48



Physics, 18.03.2020 21:49



Mathematics, 18.03.2020 21:49


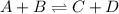
![K_{f} = \frac{[C][D]}{[A][B]}](/tpl/images/0224/4830/c1e46.png) .......... (1)
.......... (1)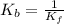
![\frac{[A][B]}{[C][D]}](/tpl/images/0224/4830/199c2.png) ............ (2)
............ (2)



