
Chemistry, 06.09.2019 17:30, PONBallfordM89
How much heat energy would be needed to raise the temperature of a 223 g sample of aluminum [(c=0.895 jig cy from 22.5°c to 55 0°c? η ο ο ο οο 10x10) not enough information given prov 40 25 11 next >

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, sophiapknight
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1



Chemistry, 22.06.2019 14:50, chem1014
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2
Do you know the correct answer?
How much heat energy would be needed to raise the temperature of a 223 g sample of aluminum [(c=0.89...
Questions in other subjects:


History, 21.06.2020 18:57


Health, 21.06.2020 18:57

Mathematics, 21.06.2020 18:57



Mathematics, 21.06.2020 18:57





 = change in temperature
= change in temperature = initial temperature =
= initial temperature = 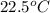
 = final temperature =
= final temperature = 







