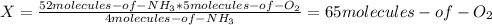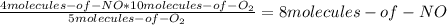Consider the following reaction: 4nh3(g) + 5o2(g) → 4no(g) + 6h2o(g) if a container were to have 10 molecules of o2 and 52 molecules of nh3 initially, how many total molecules (reactants plus products) would be present in the container after this reaction goes to completion?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, dimondqueen511
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1


Chemistry, 22.06.2019 16:50, brandiwingard
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 17:10, mikeeway33
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2
Do you know the correct answer?
Consider the following reaction: 4nh3(g) + 5o2(g) → 4no(g) + 6h2o(g) if a container were to have 10...
Questions in other subjects:

Business, 25.03.2021 07:50


English, 25.03.2021 08:00

Mathematics, 25.03.2021 08:00

Geography, 25.03.2021 08:00





Mathematics, 25.03.2021 08:00