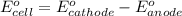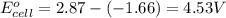Chemistry, 06.09.2019 16:20, glizbethh00
For the following electrochemical reaction: al3+(aq) + 3e -> al(s) eº = -1.66 v e° = 2.87 f2(g) + 2e -> 2f (aq) calculate eº for the cell.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, smhrosepetals
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2

Chemistry, 22.06.2019 04:30, clairajogriggsk
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 05:30, livigrace9004
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 09:30, psychocatgirl1
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone, due to its high light availability and warm water temperature
Answers: 3
Do you know the correct answer?
For the following electrochemical reaction: al3+(aq) + 3e -> al(s) eº = -1.66 v e° = 2.87 f2(g)...
Questions in other subjects:

History, 04.11.2020 19:20

Mathematics, 04.11.2020 19:20

Mathematics, 04.11.2020 19:20


Mathematics, 04.11.2020 19:20

Mathematics, 04.11.2020 19:20

Mathematics, 04.11.2020 19:20




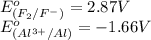
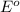 potential will always get reduced and will undergo reduction reaction. Here, fluorine will undergo reduction reaction will get reduced.
potential will always get reduced and will undergo reduction reaction. Here, fluorine will undergo reduction reaction will get reduced.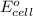 of the reaction, we use the equation:
of the reaction, we use the equation: