

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:30, kittybatch345
Is a chemical message sent by another individual.
Answers: 1

Chemistry, 22.06.2019 23:30, bxymichelle
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3
Do you know the correct answer?
13n decays with a half-life of approximately 10 min to produce 13c, a stable isotope of carbon. for...
Questions in other subjects:











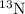 remains equal to 0.50 g. Remaining 0.50 g of mass gets converted to
remains equal to 0.50 g. Remaining 0.50 g of mass gets converted to 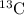 nuclide.
nuclide.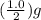 or 0.50 g.As radioactive decay of
or 0.50 g.As radioactive decay of 



