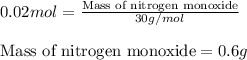Chemistry, 06.09.2019 16:10, DessAnnette2751
3cu(s) + 8 hno3(aq) → 3 cu(no3)2(aq) + 2 no(g) + 4 h20(1) a) if 5.58 g of copper(ii) nitrate, cu(no3)2, is eventually obtained, how many grams of nitrogen monoxide, no, would have formed?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, jwood287375
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 19:30, xxaurorabluexx
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 23.06.2019 00:30, clairebear66
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1
Do you know the correct answer?
3cu(s) + 8 hno3(aq) → 3 cu(no3)2(aq) + 2 no(g) + 4 h20(1) a) if 5.58 g of copper(ii) nitrate, cu(no3...
Questions in other subjects:

Spanish, 07.11.2019 10:31

History, 07.11.2019 10:31

Mathematics, 07.11.2019 10:31

Chemistry, 07.11.2019 10:31



History, 07.11.2019 10:31

Chemistry, 07.11.2019 10:31

Biology, 07.11.2019 10:31


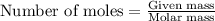 .....(1)
.....(1)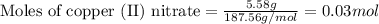
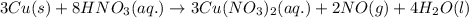
 of nitrogen monoxide is formed.
of nitrogen monoxide is formed.