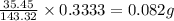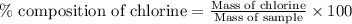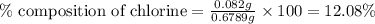The percent chloride in an unknown sample may be determined by gravimetric methods. suppose a 0.6789g sample of an unknown chloride sample was dissolved and agcl is precipitated by adding agno3 solution. the precipitate was filtered, ignited, and found to weigh 0.g. what was the percent chloride in the sample?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, VictoriaRose520
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 06:00, citlalli30
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 16:00, sassy11111515
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 16:50, shaylawaldo11
Which element is least likely to undergo a chemical reaction
Answers: 3
Do you know the correct answer?
The percent chloride in an unknown sample may be determined by gravimetric methods. suppose a 0.6789...
Questions in other subjects:


Mathematics, 18.03.2021 03:00




Chemistry, 18.03.2021 03:00


History, 18.03.2021 03:00


Mathematics, 18.03.2021 03:00