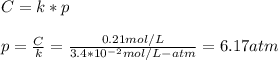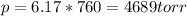Chemistry, 05.09.2019 23:30, avalianagames
Part a an unopened can of soda has an aqueous co2 concentration of 0.21 m at 25.0 °c. what is the partial pressure of the gas in the can in torr? the henry's law constant for co2 at 25 °c is 3.4 × 10−2 mol/l-atm.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:10, cordovamaria22
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1


Chemistry, 22.06.2019 12:30, americanbellabeauty
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 14:30, belindajolete
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2
Do you know the correct answer?
Part a an unopened can of soda has an aqueous co2 concentration of 0.21 m at 25.0 °c. what is the pa...
Questions in other subjects:

Mathematics, 03.04.2020 02:09

Mathematics, 03.04.2020 02:09





Mathematics, 03.04.2020 02:10


Physics, 03.04.2020 02:10

Mathematics, 03.04.2020 02:10