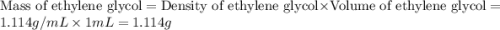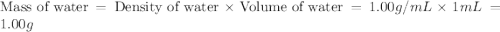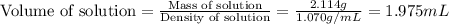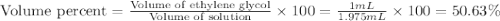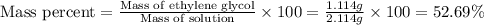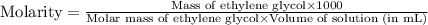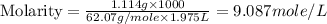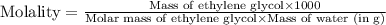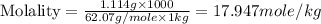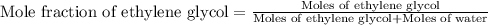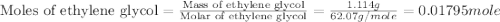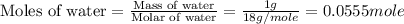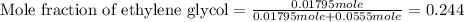An automobile antifreeze mixture is made by mixing equal volumes of ethylene glycol (d = 1.114 g/ml; m = 62.07 g/mol) and water (d = 1.00 g/ml) at 20°c. the density of the mixture is 1.070 g/ml. express the concentration of ethylene glycol as
a- volume percent
b- mass percent
c- molarity
d- molality
e- mole fraction

Answers: 1
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 16:30, jrfranckowiak
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1
Do you know the correct answer?
An automobile antifreeze mixture is made by mixing equal volumes of ethylene glycol (d = 1.114 g/ml;...
Questions in other subjects:

Mathematics, 20.04.2021 18:20



Mathematics, 20.04.2021 18:20


Mathematics, 20.04.2021 18:20

Mathematics, 20.04.2021 18:20