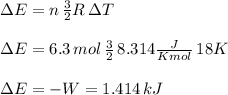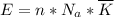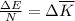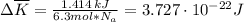Chemistry, 05.09.2019 21:20, jackchelly
The temperature of 6.30 mol of an ideal monatomic gas is raised 18.0 k in an adiabatic process. what are (a) the work w done by the gas, (b) the energy transferred as heat q, (c) the change δeint in internal energy of the gas, and (d) the change δk in the average kinetic energy per atom?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, cbelew0001ouje4i
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 16:30, danbelucio
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2


Chemistry, 23.06.2019 05:00, neidaq12345
110 g of water (specific heat = 4.184 j/g c) and 100 g of a metal sample (specific heat = 0.397 j/g c) are heated from 25 degrees c to 75 degrees c. which substance required more thermal energy?
Answers: 1
Do you know the correct answer?
The temperature of 6.30 mol of an ideal monatomic gas is raised 18.0 k in an adiabatic process. what...
Questions in other subjects:

History, 13.10.2019 00:30

Chemistry, 13.10.2019 00:30


English, 13.10.2019 00:30

Mathematics, 13.10.2019 00:30

Physics, 13.10.2019 00:30





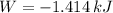 , that means, work is done on the gas, not viceversa.
, that means, work is done on the gas, not viceversa.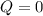 because it is an adiabatic process.
because it is an adiabatic process.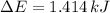
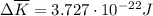 per atom
per atom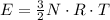 , This result can be experimentally verified or derived from statistical mechanics.
, This result can be experimentally verified or derived from statistical mechanics.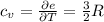
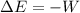
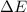 as follows:
as follows: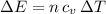 which follows from our first equation.
which follows from our first equation.