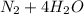Write half-reactions for the oxidation and reduction process for each of the following.
a. fe...

Chemistry, 05.09.2019 20:30, hamadehassan
Write half-reactions for the oxidation and reduction process for each of the following.
a. fe2+ + mno4 - fe3+ + mn2+
b. sn2+ + io3 - sn4+ + i-
c. s2- + no3 - s + no
d. nh3 + no2 n2 + h2o

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:10, andersonemma2222
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1



Chemistry, 23.06.2019 06:00, lanaiheart7
What are the coefficients to balance the following equation? ba+br2=babr2
Answers: 2
Do you know the correct answer?
Questions in other subjects:


Biology, 22.01.2021 04:40

Mathematics, 22.01.2021 04:40


Social Studies, 22.01.2021 04:40



Geography, 22.01.2021 04:40

Mathematics, 22.01.2021 04:40

History, 22.01.2021 04:40


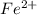 ⇒
⇒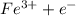
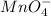 + 2
+ 2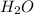 + 3
+ 3 ⇒
⇒ 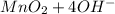
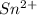 ⇒
⇒ 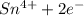
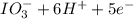 ⇒
⇒ 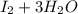
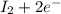 ⇒
⇒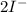
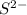 ⇒
⇒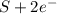
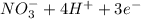 ⇒
⇒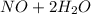
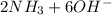 ⇒
⇒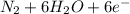
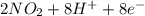 ⇒
⇒