Chemistry, 05.09.2019 20:20, kuehnkeegan
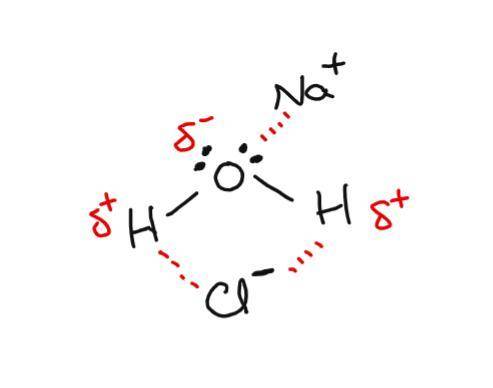
Awater molecule has polar o−h bonds that result in regions of partial positive charge (hydrogen atoms) and a region of partial negative charge (oxygen atom with lone pairs). place the na+ and cl− ions where h2o molecules are properly oriented to form ion–dipole interactions.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, lazavionadams81
Identify which properties could correspond to solids, plasmas, or both. maintain a unique shape. collide infrequently with other particles. have very high velocities. conduct electricity. protons. have a low temperature. has long-range order.
Answers: 1

Chemistry, 21.06.2019 20:40, Islandgirl67
If equal masses of the listed metals were collected , which would have a greatest volume ? a. aluminum 2.70,b. zinc7.14,c. copper 8.92,d. lead 11.34
Answers: 2

Chemistry, 21.06.2019 23:50, scavalieri2421
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3
Do you know the correct answer?
Awater molecule has polar o−h bonds that result in regions of partial positive charge (hydrogen atom...
Questions in other subjects:



Physics, 23.08.2020 02:01


Mathematics, 23.08.2020 02:01

Mathematics, 23.08.2020 02:01

Mathematics, 23.08.2020 02:01


English, 23.08.2020 02:01

History, 23.08.2020 02:01