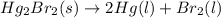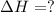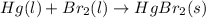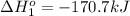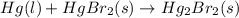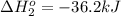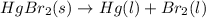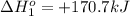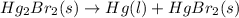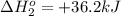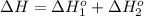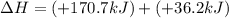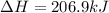Chemistry, 05.09.2019 19:30, calindaperez
Calculate δh (in kj) for the process hg2br2(s) → 2 hg(l) + br2(l) from the following information.
hg(l) + br2(l) → hgbr2(s) δh⁰298 = −170.7 kj
hg(l) + hgbr2(s) → hg2br2(s) δh⁰298 = −36.2 kj

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, bakoeboo
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 11:50, hadwell34
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 23.06.2019 03:30, LlayahHarbin
Ahelium balloon contains 16.9 l of helium at stp. how many atoms of helium are in the balloon
Answers: 1
Do you know the correct answer?
Calculate δh (in kj) for the process hg2br2(s) → 2 hg(l) + br2(l) from the following information.
Questions in other subjects:


Mathematics, 13.03.2020 02:23








Mathematics, 13.03.2020 02:23