Chemistry, 05.09.2019 18:30, itzdryoshi
Nitric acid is often manufactured from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming of natural gas, in a two-step process. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 3h2(g) > 2nh3(g) in the second step, ammonia and oxygen react to form nitric acid and water: nh3(g) + 2o2(g) > hno3(g) + h2o(g) write the net chemical equation for the production of nitric acid from nitrogen, hydrogen and oxygen. be sure your equation is balanced.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, haileywebb8
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 22.06.2019 04:00, breannaasmith1122
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 07:00, coolkid2041
Calculate the number of moles of ethane in 100 grams
Answers: 3
Do you know the correct answer?
Nitric acid is often manufactured from the atmospheric gases nitrogen and oxygen, plus hydrogen prep...
Questions in other subjects:



Mathematics, 17.04.2020 17:09








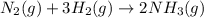 ......(1)
......(1)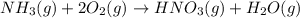 .....(2)
.....(2)