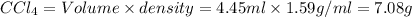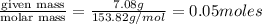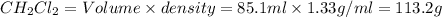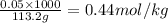Chemistry, 05.09.2019 17:30, kayelynn003
What is the molality of a solution consisting of 4.45 ml of carbon tetrachloride (ccl4; d = 1.59 g/ml) in 85.1 ml of methylene chloride (ch2cl2; d = 1.33 g/ml)?

Answers: 2
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 21:40, k3rbycalilung
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2
Do you know the correct answer?
What is the molality of a solution consisting of 4.45 ml of carbon tetrachloride (ccl4; d = 1.59 g/...
Questions in other subjects:



Mathematics, 23.08.2021 19:20

History, 23.08.2021 19:20

English, 23.08.2021 19:20

Mathematics, 23.08.2021 19:20




Mathematics, 23.08.2021 19:20


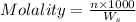
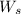 = weight of solvent in g
= weight of solvent in g