
Chemistry, 05.09.2019 17:30, bairdmatthew43
Solute s has a partition coefficient of 6.2 between water (phase 1) and hexane (phase 2). 74.0 ml solution of s in water is extracted six times with 17.0 ml of hexane. calculate the fraction of s remaining in the aqueous phase.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, psychocatgirl1
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone, due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 13:50, kelonmazon2492
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Do you know the correct answer?
Solute s has a partition coefficient of 6.2 between water (phase 1) and hexane (phase 2). 74.0 ml so...
Questions in other subjects:


Biology, 22.10.2019 22:00


Biology, 22.10.2019 22:00


History, 22.10.2019 22:00




Mathematics, 22.10.2019 22:00


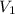 = 74.0 mL
= 74.0 mL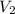 = 17.0 mL
= 17.0 mL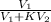
![[\frac{V_{1}}{V_{1} + KV_{2}}]^{3}](/tpl/images/0223/5422/324f2.png)
![[\frac{74.0 ml}{74.0 ml + 6.2 \times 17.0 ml}]^{3}](/tpl/images/0223/5422/2c23d.png)
![[\frac{74.0 ml}{179.4 ml}]^{3}](/tpl/images/0223/5422/f6b3c.png)




