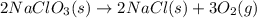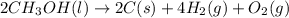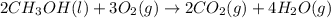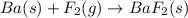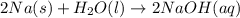Chemistry, 05.09.2019 17:20, skatingby8910
Classify each of the following reactions: i. decopositionii. combinationiii. combustion a. 2ch3oh(l)+3o2(g)→2co2(g)+4h2o(g) b. 2naclo3(s)→2nacl(s)+3o2(g) c. ba(s)+f2(g)→baf2(s) d. 2na(s)+h2o(l)→2naoh(aq) e. 2ch3oh(l)→2c(s)+4h2(g)+o2(g)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, shadekashakay
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 12:00, hannah2757
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 22:10, steven0448
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
Do you know the correct answer?
Classify each of the following reactions: i. decopositionii. combinationiii. combustion a. 2ch3oh(l)...
Questions in other subjects:

Mathematics, 16.11.2020 23:00

French, 16.11.2020 23:00

English, 16.11.2020 23:00

Mathematics, 16.11.2020 23:00


Mathematics, 16.11.2020 23:00


Mathematics, 16.11.2020 23:00

Biology, 16.11.2020 23:00