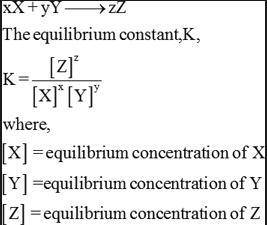
An aqueous solution of acetic acid is found to have the following equilibrium concentrations at 25 c:
[ch3cooh] = 1.65 x 10^-2 m; [h+] = 5.44 x 10^-4 m; and [ch3coo-] = 5.44 x 10^-4 m. calculate the equilibrium constant kc for the ionization of acetic acid at 25 c. the reaction is
ch3cooh -> h+ + ch3coo-

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:20, ineedhelp773
Which statement accurately describes the relationship between air pressure, air density, or altitude? as altitude increases, pressure increases. as altitude increases, air density increases. air pressure and density are lowest at sea level. denser air exerts more pressure than less dense air.
Answers: 2

Chemistry, 21.06.2019 22:30, larreanathalie3523
The diagram shows the structures of horse and cat forelimbs. what does the diagram suggest about the evolutionary relationship between these two mammals? a. they have homologous structures, indicating a common ancestor. b. they have analogous structures, indicating a common ancestor. c. they have homologous structures, indicating that they do not have a common ancestor. d. they have analogous structures, indicating that they do not have a common ancestor.
Answers: 2

Chemistry, 22.06.2019 18:10, bri9263
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3
Do you know the correct answer?
An aqueous solution of acetic acid is found to have the following equilibrium concentrations at 25 c...
Questions in other subjects:





SAT, 19.05.2021 07:50

Biology, 19.05.2021 07:50


History, 19.05.2021 08:00

Social Studies, 19.05.2021 08:00


![Kc=\frac{[H+] [CH3COO-]}{[CH3COOH]}](/tpl/images/0223/5183/bf641.png)