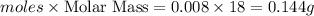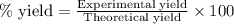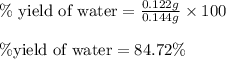Chemistry, 05.09.2019 16:20, kaymillsaps
Gaseous methane (ch4) reacts with gaseous oxygen gas (o2) to produce gaseous carbon dioxide (co2) and gaseous water (h2o) . if 0.122g of water is produced from the reaction of 0.16g of methane and 0.25g of oxygen gas, calculate the percent yield of water.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, SmolBeanPotato
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1

Chemistry, 21.06.2019 23:30, lylessd423
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2


Chemistry, 22.06.2019 15:00, raeprince9213
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1
Do you know the correct answer?
Gaseous methane (ch4) reacts with gaseous oxygen gas (o2) to produce gaseous carbon dioxide (co2) an...
Questions in other subjects:

Biology, 21.12.2019 01:31



Mathematics, 21.12.2019 01:31

Mathematics, 21.12.2019 01:31

History, 21.12.2019 01:31





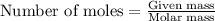 .....(1)
.....(1)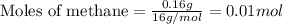
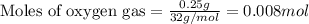
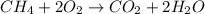
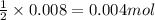 of methane
of methane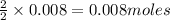 of water
of water