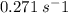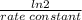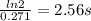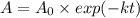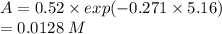Chemistry, 05.09.2019 04:10, mollykay2001p3qo0j
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1.
(a) what is the half-life for this reaction?
(b) if you start with 0.052 m i2 at this temperature, how much will remain after 5.16 s assuming that the iodine atoms do not recombine to form i2?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, rosetoheart2
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1



Chemistry, 22.06.2019 16:30, montanolumpuy
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
Do you know the correct answer?
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of...
Questions in other subjects:

Mathematics, 23.07.2019 23:00



Social Studies, 23.07.2019 23:00






English, 23.07.2019 23:00