
Chemistry, 05.09.2019 00:20, kimloveswim
The combustion of titanium with oxygen produces titanium dioxide: ti(s) + o2(g) → tio2(s) when 2.060 g of titanium is combusted in a bomb calorimeter, the temperature of the calorimeter increases from 25.00 °c to 91.60 °c. in a separate experiment, the heat capacity of the calorimeter is measured to be 9.84 kj/k. the heat of reaction for the combustion of a mole of ti in this calorimeter is kj/mol.

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 05:20, jtingley0502
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1
Do you know the correct answer?
The combustion of titanium with oxygen produces titanium dioxide: ti(s) + o2(g) → tio2(s) when 2.06...
Questions in other subjects:


Mathematics, 24.04.2020 22:14

English, 24.04.2020 22:14

Biology, 24.04.2020 22:14


History, 24.04.2020 22:14






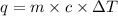
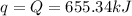
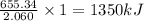 of heat
of heat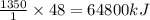 of heat
of heat



