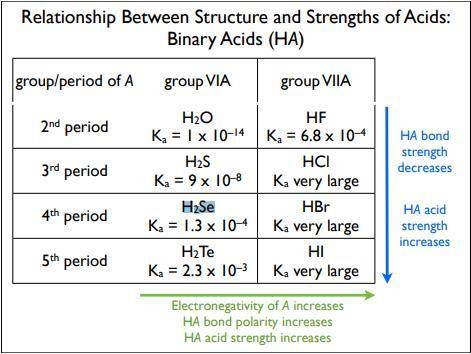
Chemistry, 04.09.2019 22:30, tiamaharaj
Enter the chemical formula of a binary molecular compound of hydrogen and a group 6a element that can reasonably be expected to be less acidic in aqueous solution than h2se, e. g. have a smaller ka .

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 08:40, kellymcdow5135
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 12:30, hayleyconsole
Nebulae are enormous clouds in outer space. they are made mostly of hydrogen gas, helium gas, and dust. some nebulae glow brightly, while others do not. the stars that people see are huge, bright balls of glowing gas. they are made mostly of hydrogen and helium. which statement correctly describes other ways in which nebulae and stars are different? a. stars can form inside a nebula but a nebula can never be produced by any star. b. a star always has a higher density than a nebula. c. stars can never form inside a nebula but a nebula can be produced by any star. d. a nebula always has a higher density than a star.
Answers: 3
Do you know the correct answer?
Enter the chemical formula of a binary molecular compound of hydrogen and a group 6a element that ca...
Questions in other subjects:




Mathematics, 21.08.2019 11:10




English, 21.08.2019 11:10

Mathematics, 21.08.2019 11:10

English, 21.08.2019 11:10