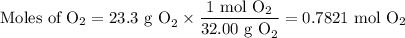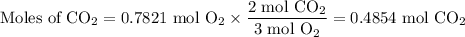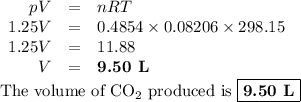The combustion of ethanol (c2h5oh) takes place by the following reaction equation.
c2h5oh (l)...

Chemistry, 04.09.2019 22:20, bionicboy03120440
The combustion of ethanol (c2h5oh) takes place by the following reaction equation.
c2h5oh (l) + 3 o2 (g) → 2 co2 (g) + 3 h2o (g)
what is the volume of co2 gas produced by the combustion of excess ethanol by 23.3 grams of o2 gas at 25°c and 1.25 atm?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, mvtthewisdead
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1


Chemistry, 22.06.2019 19:30, toriabrocks
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 23.06.2019 02:00, Turtlelover05
Pinene is an unsaturated hydrocarbon found in pine resin. if pinene has m+ = 136 and contains 1 double bond(s) and 2 ring(s); what is its molecular formula? enter the formula in the form ch first, then all other atoms in alphabetical order; do not use subscripts. the formula is case-sensitive
Answers: 3
Do you know the correct answer?
Questions in other subjects:


Biology, 02.05.2021 01:40



Mathematics, 02.05.2021 01:40

Arts, 02.05.2021 01:40

Chemistry, 02.05.2021 01:40

Mathematics, 02.05.2021 01:40

Law, 02.05.2021 01:40