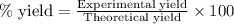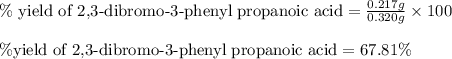Suppose a student started with 155.0 mg of trans-cinnamic acid, 458 mg of pyridinium tribromide, and 2.35 ml of glacial acetic acid. after the reaction and workup, the student ended up with 0.2170 g of brominated product. calculate the student\'s theoretical and percent yields.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:10, amuijakobp78deg
Agas mixture with a total pressure of 745 mmhg contains each of the following gases at the indicated partial pressures: co2, 245 mmhg ; ar, 119 mmhg ; and o2, 163 mmhg . the mixture also contains helium gas. part a what is the partial pressure of the helium gas? phe p h e = nothing mmhg request answer part b what mass of helium gas is present in a 10.2-l sample of this mixture at 283 k ? m m = nothing g request answer
Answers: 1


Chemistry, 22.06.2019 10:40, trinityanne1738
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 11:30, charles8527
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2
Do you know the correct answer?
Suppose a student started with 155.0 mg of trans-cinnamic acid, 458 mg of pyridinium tribromide, and...
Questions in other subjects:


History, 05.11.2020 01:40


English, 05.11.2020 01:40

History, 05.11.2020 01:40


History, 05.11.2020 01:40


Mathematics, 05.11.2020 01:40

Biology, 05.11.2020 01:40


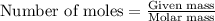 .....(1)
.....(1)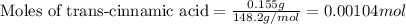
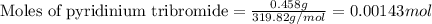
![\text{trans-cinnamic acid}+\text{pyridinium tribromide}\xrightarrow[]{CH_3COOH}\text{2,3-dibromo-3-phenyl propanoic acid}](/tpl/images/0222/4310/95be0.png)
 of pyridinium tribromide
of pyridinium tribromide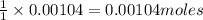 of 2,3-dibromo-3-phenyl propanoic acid
of 2,3-dibromo-3-phenyl propanoic acid