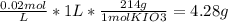Chemistry, 04.09.2019 03:20, JosefineRubino2204
You wish to prepare 1 l of a 0.02 m potassium iodate solution. you require that the final concentration be within 1% of 0.02 m and that the concentration must be known accurately to the fourth decimal place. how would you prepare this solution? specify the glassware you would use, the accuracy needed for the balance, and the ranges of acceptable masses of kio3 that could be used.(a) to make this solution (ideally) you would need grams of potassium iodide dissolved in enough water to make up 1 l of solution. fill in the blank(b)what is the least accurate balance that could be used to make this solution?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, latoyatuggle23
Endeleev saw trends in the physical and chemical properties of elements when he organized them by
Answers: 2


Chemistry, 22.06.2019 21:30, sarah192002
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1

Chemistry, 23.06.2019 00:10, Rubendelarosa1529
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3
Do you know the correct answer?
You wish to prepare 1 l of a 0.02 m potassium iodate solution. you require that the final concentrat...
Questions in other subjects:



History, 03.03.2022 04:40




English, 03.03.2022 04:40


Mathematics, 03.03.2022 04:40