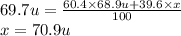Chemistry, 04.09.2019 03:20, hosteenimport21
The element gallium has an atomic mass of 69.7 u and consists of two stable isotopes gallium-69 and gallium-71. the isotope gallium-69 has a mass of 68.9 u and a percent natural abundance of 60.4 %. the isotope gallium-71 has a percent natural abundance of 39.6 %. what is the mass of gallium-71

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, quantamagic
Word equation for k(s)+h2o(l) yield koh(aq) + h2(g)
Answers: 1

Chemistry, 22.06.2019 13:00, cnfndbxbfbdb2031
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Do you know the correct answer?
The element gallium has an atomic mass of 69.7 u and consists of two stable isotopes gallium-69 and...
Questions in other subjects:

Spanish, 05.05.2021 19:40

Social Studies, 05.05.2021 19:40

Mathematics, 05.05.2021 19:40

Mathematics, 05.05.2021 19:40

Social Studies, 05.05.2021 19:40

English, 05.05.2021 19:40




Computers and Technology, 05.05.2021 19:40


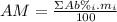
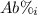 : percent natural abundance of each isotope
: percent natural abundance of each isotope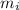 : mass of each isotope
: mass of each isotope