Chemistry, 04.09.2019 00:10, ashley5196
Sulfuric acid (h2so4) is prepared commercially from elemental sulfur using the contact process. in a typical sequence of reactions, the sulfur is first burned: s + o2 → so2 , then it is converted to so3 using a catalyst: 2 so2 + o2 → 2 so3 . the resulting so3 is reacted with water to produce the desired product: so3 + h2o → h2so4 . how much sulfuric acid could be prepared from 83 moles of sulfur? answer in units of g.

Answers: 1
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 10:00, nana54muller
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1
Do you know the correct answer?
Sulfuric acid (h2so4) is prepared commercially from elemental sulfur using the contact process. in a...
Questions in other subjects:

Mathematics, 20.12.2020 14:00

Chemistry, 20.12.2020 14:00


English, 20.12.2020 14:00



Computers and Technology, 20.12.2020 14:00


Computers and Technology, 20.12.2020 14:00

Mathematics, 20.12.2020 14:00


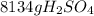
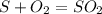
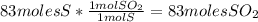
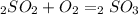
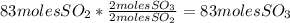
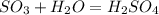
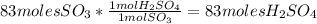
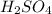 :
: