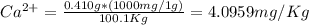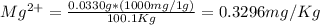Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, alydiale584
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 09:00, valeriekbueno
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

Chemistry, 22.06.2019 13:30, kassandrarosario1115
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 22:10, zwbaby3693
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3
Do you know the correct answer?
Wastewater from a cement factory contains 0.410 g of ca2+ ion and 0.0330 g of mg2+ ion per 100.0 l o...
Questions in other subjects:


English, 16.04.2021 21:30

Mathematics, 16.04.2021 21:30


Mathematics, 16.04.2021 21:30


Mathematics, 16.04.2021 21:30

Mathematics, 16.04.2021 21:30

Mathematics, 16.04.2021 21:30


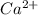 = 4.0959 ppm
= 4.0959 ppm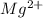 = 0.3296 ppm
= 0.3296 ppm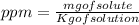
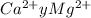
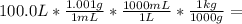 = 100.1 kg
= 100.1 kg