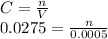Chemistry, 03.09.2019 21:30, champions2k19
A25.0-ml sample containing cu2+ gave an instrument signal of 25.2 units (corrected for a blank). when exactly 0.500 ml of 0.0275 m cu(no3)2 was added to the solution, the signal increased to 45.1 units. calculate the molar concentration of cu2+ assuming that the signal was directly proportional to the analyte concentration. skoog, douglas a.. principles of instrumental analysis (p. 20). brooks cole. kindle edition.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:00, clairebear66
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 14:00, emilyproce
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1
Do you know the correct answer?
A25.0-ml sample containing cu2+ gave an instrument signal of 25.2 units (corrected for a blank). whe...
Questions in other subjects:


Mathematics, 02.12.2021 19:40


Mathematics, 02.12.2021 19:40

Mathematics, 02.12.2021 19:40