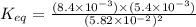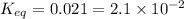2hf(g)< > h2(g)+f2(g)
consider the reaction.
at equilibrium at 600 k, the concentrations are as follows.[hf] = 5.82 x 10^-2 m[h2] = 8.4 x 10^-3 m[f2] = 8.4 x 10^-3 mwhat is the value of keq for the reaction expressed in scientific notation? a. 2.1 x 10^-2b. 2.1 x 10^2c. 1.2 x 10^3d. 1.2 x 10^-3

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:40, jeffcarpenter
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1



Do you know the correct answer?
2hf(g)< > h2(g)+f2(g)
consider the reaction.
at equilibrium at 600 k, the concent...
consider the reaction.
at equilibrium at 600 k, the concent...
Questions in other subjects:

Spanish, 18.12.2020 01:30


Physics, 18.12.2020 01:30


Mathematics, 18.12.2020 01:30


Mathematics, 18.12.2020 01:30

Mathematics, 18.12.2020 01:30



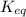 for the following reaction will be,
for the following reaction will be, 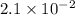
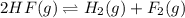
![K_{eq}=\frac{[H_2][F_2]}{[HF]^2}](/tpl/images/0222/0267/d9c6c.png)