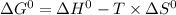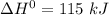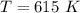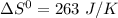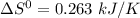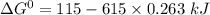Chemistry, 03.09.2019 05:20, ayoismeisalex
Give the values of δho, δso, and t, determine δgo and indicate if the reaction is spontaneous. δho = 115 kj , δso = 263 j/k , and t = 615k δgo= kj spontaneous? yes or no

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, wkalpakchi
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1


Chemistry, 23.06.2019 04:20, tyrickdavis1
The equation below shows a chemical reaction. a + b + heat —> c + d according to the law of conservation of energy, which statement is true? a. the reactants absorb heat because they have less energy than the products. b. the products release heat because they have more energy than the reactants. c. the reactants generate heat because they have more energy than the products. d. the products require heat to form because they have less energy than the reactants.
Answers: 1
Do you know the correct answer?
Give the values of δho, δso, and t, determine δgo and indicate if the reaction is spontaneous. δho =...
Questions in other subjects:

Mathematics, 11.06.2020 07:57

History, 11.06.2020 07:57




Computers and Technology, 11.06.2020 07:57

History, 11.06.2020 07:57

Health, 11.06.2020 07:57

Mathematics, 11.06.2020 07:57


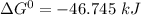
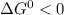 , the reaction is spontaneous.
, the reaction is spontaneous.