
Which of the following states is true? (a) q does not change with temperature. (b) q is the same as keq when a reaction is at equilibrium. (c) key does not change with temperature, whereas q is temperature dependent. (d) k does not depend on the concentration or partial pressures of reaction components. (e) q does not depend on the concentrations or partial pressures of reaction components.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:50, awesomegamergurl13
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 15:00, kandi2565
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Do you know the correct answer?
Which of the following states is true? (a) q does not change with temperature. (b) q is the same as...
Questions in other subjects:


Mathematics, 30.04.2021 01:20



Chemistry, 30.04.2021 01:20

Mathematics, 30.04.2021 01:20

Mathematics, 30.04.2021 01:20

Mathematics, 30.04.2021 01:20


English, 30.04.2021 01:20


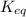 is the equilibrium constant and it is also equal to the concentration of products divided by the concentration of reactants. Also,
is the equilibrium constant and it is also equal to the concentration of products divided by the concentration of reactants. Also, 



