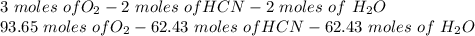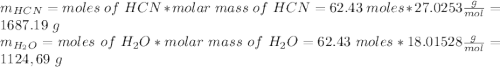Hydrogen cyanide is produced industrially from the reaction of gaseous ammonia, oxygen, and methane: 2nh3 1g2 1 3o2 1g2 1 2ch4 1g2 h 2hcn1g2 1 6h2o1g2 if 5.00 3 103 kg each of nh3, o2, and ch4 are reacted, what mass of hcn and of h2o will be produced, assuming 100% yield?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:00, batoolishak7475
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 23.06.2019 15:40, mackdoolittle1
Glucose (c6h12o6) is the simple sugar that plants make. what is the total number of atoms in glucose? 1 3 24 144
Answers: 1

Do you know the correct answer?
Hydrogen cyanide is produced industrially from the reaction of gaseous ammonia, oxygen, and methane:...
Questions in other subjects:

Mathematics, 04.09.2020 07:01



Mathematics, 04.09.2020 07:01

Computers and Technology, 04.09.2020 07:01


Mathematics, 04.09.2020 07:01


Physics, 04.09.2020 07:01

History, 04.09.2020 07:01


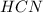 and 1124,69 g of
and 1124,69 g of 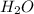
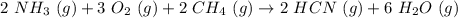
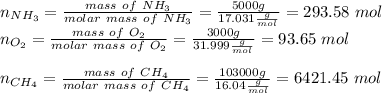
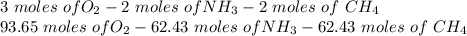
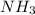 and
and 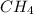 to consume the oxygen. In case there wasn´t enough of one of the other reactants, that one would be the limiting one.
to consume the oxygen. In case there wasn´t enough of one of the other reactants, that one would be the limiting one.