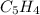Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:10, puchie1225
Cobalt-60 is an artificial radioisotope that is produced in a nuclear reactor and is used as a gamma-ray source in the treatment of certain types of cancer. if the wavelength of the gamma radiation from a cobalt-60 source is 1.00 × 10-3 nm, calculate the energy of a photon of this radiation.
Answers: 2

Chemistry, 22.06.2019 01:30, adrian08022
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 06:00, jwood287375
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Do you know the correct answer?
The principal component of mothballs is naphthalene, a compound with the molecular mass of about 130...
Questions in other subjects:

Mathematics, 13.07.2019 23:00







Mathematics, 13.07.2019 23:00

Mathematics, 13.07.2019 23:00


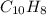 - Molecular formula
- Molecular formula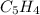 - Empirical formula
- Empirical formula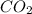 = 10.3 × 10⁻³ g /44.01 g/mol = 0.2340×10⁻³ moles
= 10.3 × 10⁻³ g /44.01 g/mol = 0.2340×10⁻³ moles