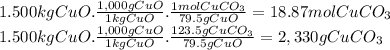Chemistry, 02.09.2019 22:10, BlueExorcistReaper
Determine the number of moles and mass requested for each reaction in exercise 4.42.
refer to exercise 4.42.
write the balanced equation, then outline the steps necessary determine the information equation in each of the following:
(a) the number of moles than the mass of the chlorine cl2 required to react with 10.0 g of sodium metal na to produce sodium chloride nacl
(b) the number of moles and the mass of the oxygen formed by the decomposition of 1.252 gram of mercury (ii) oxide
(c)the number of the moles and the mass of the sodium nitrate nano3 required to produce 128 gram of oxygen (nano2 is the other product)
(d)the number of moles and the mass of the carbon dioxide formed by the combustion of 20.0 kg of carbon in excess of oxygen
(e)the number of moles and the mass of the copper(ii) carbonate needed to produce 1.500 kg of copper ii oxide (co2 is the other product)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, EMQPWE
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1


Chemistry, 23.06.2019 07:00, phancharamachasm
Determine the length of the object shown. 97.8 mm 97.80 mm 97 mm 98 mm
Answers: 1

Chemistry, 23.06.2019 09:00, cyni
Which question could be best answered using the process of scientific inquiry? do different plates have different rock compositions? why did it take so long to develop the theory of plate tectonics? what are different cultural myths caused by plate tectonics? do plates move intentionally to cause volcanic eruptions?
Answers: 3
Do you know the correct answer?
Determine the number of moles and mass requested for each reaction in exercise 4.42.
refer to...
refer to...
Questions in other subjects:


Mathematics, 14.05.2021 16:40

Mathematics, 14.05.2021 16:40

Spanish, 14.05.2021 16:40



Chemistry, 14.05.2021 16:40



Mathematics, 14.05.2021 16:40


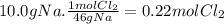
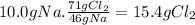
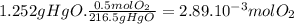
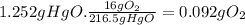
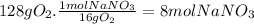
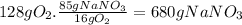
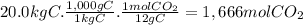
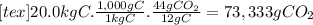 " alt="20.0kgC.\frac{1,000gC}{1kgC} .\frac{44gCO_{2}}{12gC} =73,333gCO_{2" />" />
" alt="20.0kgC.\frac{1,000gC}{1kgC} .\frac{44gCO_{2}}{12gC} =73,333gCO_{2" />" />